How To Calculate Osmotic Pressure? Simple Formula Guide

Osmotic pressure is a critical concept in chemistry and biology, describing the pressure required to prevent the flow of solvent molecules through a semipermeable membrane that separates a solution from pure solvent. The calculation of osmotic pressure is essential in various fields, including physiology, where it plays a significant role in maintaining fluid balance within cells, and in industrial applications, such as desalination and wastewater treatment. In this article, we will delve into the simple formula guide for calculating osmotic pressure, exploring its underlying principles and practical applications.
Understanding Osmotic Pressure

Osmotic pressure is directly related to the concentration of solutes in a solution. According to the van ’t Hoff equation, the osmotic pressure (π) of a solution is given by the formula π = cRT, where c is the concentration of the solute in moles per liter (molarity), R is the ideal gas constant, and T is the temperature in Kelvin. This equation is a simplified version of the ideal gas law, adapted for solutions, and it provides a straightforward method for calculating the osmotic pressure of a solution, given its concentration and temperature.
The van ’t Hoff Equation
The van ’t Hoff equation, π = cRT, is fundamental in calculating osmotic pressure. Here, the concentration © of the solute is expressed in moles per liter (M), the gas constant ® is approximately 0.0821 L·atm/(mol·K), and the temperature (T) is in Kelvin. To apply this equation, one must know the molarity of the solution and the temperature at which the osmotic pressure is being measured. For instance, if you have a 1 M solution of sodium chloride (NaCl) at 25°C (298 K), you can calculate the osmotic pressure using the van ’t Hoff equation.
| Component | Value |
|---|---|
| Concentration (c) | 1 M |
| Gas Constant (R) | 0.0821 L·atm/(mol·K) |
| Temperature (T) | 298 K |

Plugging these values into the equation gives π = 1 M * 0.0821 L·atm/(mol·K) * 298 K = 24.45 atm. This result indicates that the osmotic pressure of a 1 M solution of NaCl at 25°C is approximately 24.45 atmospheres.
Calculating Osmotic Pressure for Electrolytes
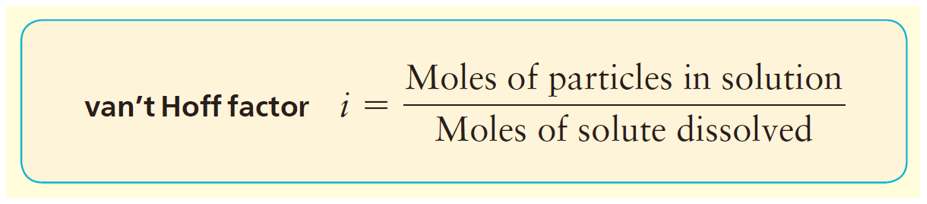
For solutions containing electrolytes, which dissociate into more than one particle (e.g., NaCl dissociates into Na+ and Cl-), the calculation of osmotic pressure requires adjustment. The van ’t Hoff factor (i) is used to account for the increase in the number of particles in solution due to dissociation. The adjusted equation becomes π = icRT, where i is the van ’t Hoff factor. For a 1:1 electrolyte like NaCl, i is approximately 2, reflecting the dissociation into two ions. This adjustment is crucial for accurately predicting the osmotic pressure of electrolyte solutions.
Practical Applications
The calculation of osmotic pressure has numerous practical applications. In medical settings, understanding osmotic pressure is vital for managing fluid balance and osmoregulation in the body. In industrial processes, such as reverse osmosis for water purification, knowing how to calculate osmotic pressure is essential for designing and operating the systems efficiently. Additionally, in food preservation, osmotic pressure plays a role in techniques like osmotic dehydration, which helps to remove water from foods, thus preserving them.
| Application | Description |
|---|---|
| Medical | Fluid balance and osmoregulation in the body |
| Industrial | Water purification through reverse osmosis |
| Food Preservation | Osmotic dehydration to remove water from foods |
In conclusion, calculating osmotic pressure using the van 't Hoff equation and understanding its applications are essential skills in chemistry, biology, and related fields. Whether for academic pursuits, industrial applications, or simply to appreciate the intricacies of natural processes, grasping the concept of osmotic pressure and how to calculate it provides valuable insights into the behavior of solutions and their role in our world.
What is osmotic pressure, and why is it important?
+Osmotic pressure is the pressure required to prevent the flow of solvent molecules through a semipermeable membrane that separates a solution from pure solvent. It’s crucial in various fields, including physiology for maintaining fluid balance and in industrial applications like desalination.
How do you calculate osmotic pressure for a solution of an electrolyte?
+To calculate the osmotic pressure of an electrolyte solution, you use the adjusted van ’t Hoff equation, π = icRT, where i is the van ’t Hoff factor that accounts for the dissociation of the electrolyte into multiple particles.
What are some practical applications of calculating osmotic pressure?
+Practical applications include medical settings for managing fluid balance, industrial processes like reverse osmosis for water purification, and food preservation techniques such as osmotic dehydration.