Orbital For Osmium

Osmium, a hard, brittle, blue-gray or blue-black transition metal in the platinum group, has a unique set of properties that make it an interesting subject for study. With the atomic number 76, osmium is the densest naturally occurring element, known for its extreme hardness and corrosion resistance. The orbital configuration of osmium is a critical aspect of understanding its chemical and physical properties.
Introduction to Orbital Configuration
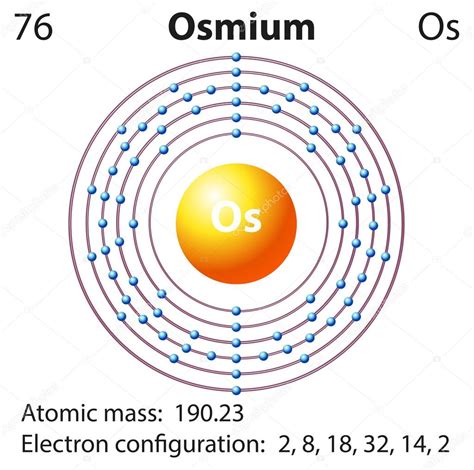
The orbital configuration of an element describes the way electrons are distributed among the available orbitals. For osmium, the electronic configuration is [Xe] 4f14 5d4 6s2. This configuration indicates that osmium has a full outer energy level, with 14 electrons in the 4f orbitals, 4 electrons in the 5d orbitals, and 2 electrons in the 6s orbitals. Understanding the orbital configuration of osmium is essential for predicting its chemical behavior and reactivity.
Atomic Orbitals and Electron Configuration
The atomic orbitals of osmium are described using the s, p, d, and f notation. The s orbitals are spherical in shape, while the p orbitals are dumbbell-shaped. The d orbitals have a four-leaf clover shape, and the f orbitals have a more complex shape with six lobes. The electron configuration of osmium can be written as 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d4 6s2. This configuration shows that osmium has a full outer energy level, with all the electrons paired in the available orbitals.
| Orbital | Electron Capacity | Electron Occupancy |
|---|---|---|
| 1s | 2 | 2 |
| 2s | 2 | 2 |
| 2p | 6 | 6 |
| 3s | 2 | 2 |
| 3p | 6 | 6 |
| 3d | 10 | 10 |
| 4s | 2 | 2 |
| 4p | 6 | 6 |
| 4d | 10 | 10 |
| 5s | 2 | 2 |
| 5p | 6 | 6 |
| 4f | 14 | 14 |
| 5d | 10 | 4 |
| 6s | 2 | 2 |

Chemical Properties and Reactivity
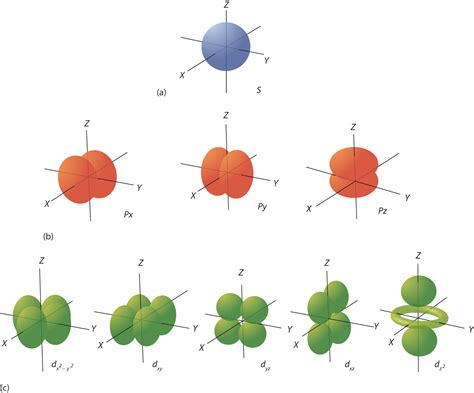
Osmium is a highly reactive metal, but its reactivity is influenced by its orbital configuration. The presence of 14 electrons in the 4f orbitals and 4 electrons in the 5d orbitals makes osmium prone to forming compounds with other elements. Osmium is known to form a range of compounds, including osmium tetroxide (OsO4), osmium dioxide (OsO2), and osmium carbide (OsC). The chemical properties of osmium are also influenced by its ability to form bonds with other elements, including carbon, nitrogen, and oxygen.
Applications of Osmium
Despite its highly reactive nature, osmium has several important applications. One of the main uses of osmium is in the production of fountain pen nibs, where its extreme hardness and corrosion resistance make it an ideal material. Osmium is also used in electrical contacts, where its high melting point and conductivity make it a valuable component. Additionally, osmium is used in the production of implantable medical devices, where its biocompatibility and corrosion resistance are essential.
- Fountain pen nibs
- Electrical contacts
- Implantable medical devices
- Catalysts
- High-temperature alloys
What is the orbital configuration of osmium?
+The orbital configuration of osmium is [Xe] 4f14 5d4 6s2, indicating that osmium has a full outer energy level with 14 electrons in the 4f orbitals, 4 electrons in the 5d orbitals, and 2 electrons in the 6s orbitals.
What are the main applications of osmium?
+Osmium is used in the production of fountain pen nibs, electrical contacts, implantable medical devices, catalysts, and high-temperature alloys. Its extreme hardness, corrosion resistance, and high melting point make it a valuable component in these applications.
What is the electron configuration of osmium?
+The electron configuration of osmium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d4 6s2, indicating that osmium has a full outer energy level with all the electrons paired in the available orbitals.


